The Impact of Changing Electrolyte Components on Battery Performance and Safety
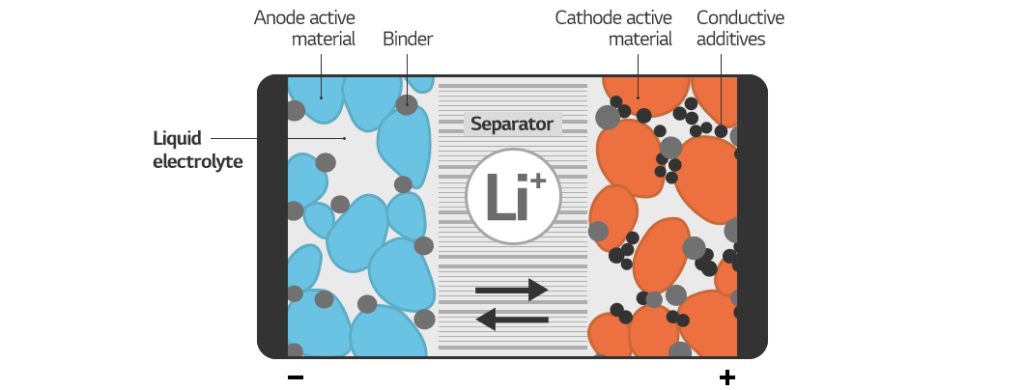
The electrolyte is a critical component of any battery, acting as the medium through which ions travel between the cathode and anode during charging and discharging. The composition of the electrolyte significantly influences battery performance, lifespan, safety, and cost. Consequently, researchers and manufacturers are constantly exploring novel electrolyte formulations and the effects of changing electrolyte components to enhance battery technology. This article delves into the various aspects of how altering these components affects overall battery characteristics.
Understanding Electrolyte Basics
Before examining the impact of changing electrolyte components, it’s crucial to understand the fundamental role of the electrolyte. Typically, an electrolyte consists of a solvent, a salt, and additives. The solvent dissolves the salt, creating a conductive medium. The salt provides the ions necessary for charge transfer. Additives are included to improve specific properties, such as thermal stability, ionic conductivity, or electrode passivation.
- Solvent: Common solvents include organic carbonates (e.g., ethylene carbonate, dimethyl carbonate), ethers, and ionic liquids.
- Salt: Lithium salts (e.g., LiPF6, LiTFSI, LiBOB) are frequently used in lithium-ion batteries.
- Additives: These can range from film-forming agents (e.g., vinylene carbonate) to overcharge protection agents.
Impact on Battery Performance
Ionic Conductivity
One of the primary goals of changing electrolyte components is to optimize ionic conductivity. Higher ionic conductivity translates to lower internal resistance, enabling faster charging and discharging rates. The choice of solvent and salt significantly affects conductivity. For instance, ionic liquids often exhibit high ionic conductivity, but their viscosity can limit ion transport in some cases. Researchers often experiment with solvent mixtures to balance conductivity, viscosity, and electrochemical stability.
Electrochemical Stability
Electrochemical stability refers to the electrolyte’s ability to withstand oxidation and reduction reactions at the electrodes. If the electrolyte decomposes, it can lead to the formation of undesirable byproducts, increased resistance, and reduced battery lifespan. Changing electrolyte components to enhance electrochemical stability is essential, especially for high-voltage batteries. Additives such as fluoroethylene carbonate (FEC) are frequently used to form a stable solid electrolyte interphase (SEI) layer on the anode surface, preventing further electrolyte decomposition.
Operating Temperature Range
The operating temperature range of a battery is heavily influenced by the electrolyte. Some electrolytes may freeze or decompose at extreme temperatures, leading to performance degradation or safety hazards. Changing electrolyte components can widen the operating temperature range. For example, using different salt concentrations or incorporating specific additives can improve low-temperature performance. High-temperature performance can be enhanced by using thermally stable solvents and salts, such as those found in solid-state electrolytes.
Impact on Battery Safety
Flammability and Volatility
Traditional liquid electrolytes are often flammable and volatile, posing a significant safety risk. Thermal runaway, a phenomenon where the battery rapidly overheats, can lead to fire or explosion. Changing electrolyte components to non-flammable or less volatile materials is a major area of research. Ionic liquids and solid-state electrolytes are promising alternatives in this regard. Furthermore, adding flame retardants to the electrolyte can mitigate the risk of fire.
Electrochemical Window
The electrochemical window defines the voltage range within which the electrolyte is stable. Exceeding this range can lead to electrolyte decomposition and gas generation, increasing the risk of swelling and rupture. Changing electrolyte components to widen the electrochemical window is crucial for developing high-voltage batteries. Additives that form protective layers on the electrodes can prevent electrolyte oxidation or reduction, effectively extending the electrochemical window.
Overcharge and Overdischarge Protection
Overcharging or overdischarging a battery can cause irreversible damage and pose safety risks. Changing electrolyte components to include additives that provide overcharge or overdischarge protection can enhance battery safety. Redox shuttles, for example, can prevent overcharging by oxidizing at the cathode before the electrolyte decomposes. Similarly, additives that form a protective layer on the anode can prevent overdischarge damage.
Emerging Trends in Electrolyte Development
Solid-State Electrolytes
Solid-state electrolytes (SSEs) are gaining increasing attention due to their potential to improve both battery performance and safety. SSEs are non-flammable and can enable the use of high-energy-density electrode materials, such as lithium metal. Changing electrolyte components from liquid to solid requires significant materials science and engineering efforts. Different types of SSEs include:
- Ceramic electrolytes: Offer high ionic conductivity and electrochemical stability.
- Polymer electrolytes: Flexible and easy to process, but typically have lower ionic conductivity.
- Composite electrolytes: Combine the advantages of ceramic and polymer electrolytes.
Ionic Liquids
Ionic liquids (ILs) are salts that are liquid at or near room temperature. They exhibit high ionic conductivity, low volatility, and wide electrochemical windows, making them attractive alternatives to traditional organic electrolytes. Changing electrolyte components to ILs can improve battery safety and performance, especially at high temperatures. However, ILs can be expensive and may have high viscosity, which can limit ion transport.
Redox-Active Electrolytes
Redox-active electrolytes contain redox-active molecules that can participate in charge transfer reactions. These electrolytes can increase the energy density and power density of batteries. Changing electrolyte components to incorporate redox-active molecules requires careful selection and optimization of the redox couple. The redox potential, stability, and solubility of the redox-active molecules are critical factors to consider.
The Future of Battery Technology
The quest for better batteries is ongoing, driven by the increasing demand for electric vehicles, portable electronics, and grid-scale energy storage. Changing electrolyte components is a crucial aspect of this quest. Researchers are continuously exploring novel materials, formulations, and architectures to enhance battery performance, safety, and cost. [See also: Advanced Battery Materials] The future of battery technology will likely involve a combination of innovative electrolyte designs and advanced electrode materials.
Conclusion
In conclusion, changing electrolyte components is a multifaceted approach to improving battery technology. By carefully selecting and optimizing the solvent, salt, and additives, it is possible to enhance ionic conductivity, electrochemical stability, operating temperature range, and safety. Emerging trends such as solid-state electrolytes, ionic liquids, and redox-active electrolytes hold great promise for the future of battery technology. As research and development efforts continue, we can expect to see further advancements in battery performance, safety, and sustainability. The continuous exploration of changing electrolyte components is vital for meeting the ever-growing energy storage demands of our society.
